On 25 Feb.,2021, Influenza Vaccine, Live, Nasal, Freeze-dried produced by Changchun BCHT Biotechnology Co. (BCHT) has been approved by the National Medical Products Administration, marking the beginning of the era of nasal spray influenza vaccine in China.
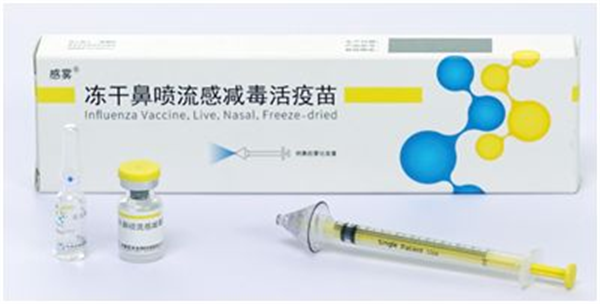
Influenza Vaccine, Live, Nasal, Freeze-dried produced by BCHT is a cooperative project with World Health Organization (WHO), and has joined Global Action Plan for Influenza Vaccines. The live attenuated vaccine is produced by the cold-adapted influenza strain provided by WHO, and the live attenuated strain can only survive in the nasal cavity and respiratory tract of human body, where the temperature is below 37℃。
After technology transfer from Russia, BCHT made two important technology improvements to the vaccine:
First one is use the SPF eggs to culture the virus;
Second one is further improved the vaccine safety by purification.
Different from the inactivated influenza vaccine, the cold-adapted influenza virus doesn’t need to be inactivated and split during the manufacturing process, thus there has no inactivator and split agent.
Influenza Vaccine, Live, Nasal, Freeze-dried contains 3 type influenza virus, and suits for 3 to 17 years old age group. It is reported that vaccination of Influenza Vaccine, Live, Nasal, Freeze-dried can induce cross-protection to some extent (which means it can protect the body from infection by virus contained in the vaccine and other influenza virus type).
In addition, the vaccine can not only induce humoral immunity, which the inactivated influenza vaccine does, but also induce cellular immunity, which the inactivated influenza vaccine does not, and the vaccine can induce mucosal immunity directly in nasal cavity, which can establish the first barrier against influenza for the body.